Microscopy images reveal the process of cellular infection by the Sars-CoV-2
24/05/2022
Maíra Menezes (IOC/Fiocruz)
A study led by the Oswaldo Cruz Institute (IOC/Fiocruz) registered, in images, the step-by-step of the infection process caused by the coronavirus (Sars-CoV-2) in Vero cells, obtained from monkey kidneys and widely used in research to understand pathogen biology and to develop treatments. Published in Viruses magazine, the work shows how the virus invades the cell, identifies the compartments where important steps of viral replication occur, and captures the moment new viral particles are released, ready to invade other cells. The photos and videos also show details on the alterations caused by the coronavirus in the cellular structure, which favors the infectious process and often results in damage to cellular functions.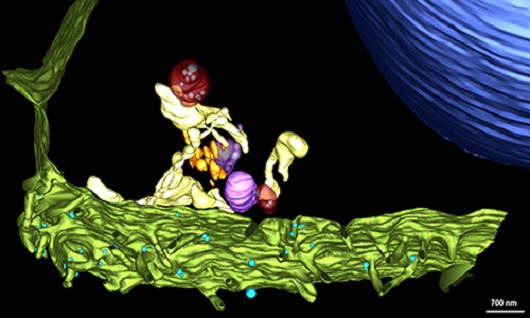
(Image: Barreto-Vieira et al; Viruses)
The three-dimensional model shows the structures involved in the infection process by the Sars-CoV-2. Viral particles (shown in blue) can be seen adhering to the cell membrane (green). Within the cell, copies of the viral genome are produced inside double-membrane vesicles (red). The synthesis of new viral particles is concluded in intermediary vesicles (purple), which later shift position and merge with the cell membrane, releasing the viral progeny (viral particles that make up the virus’s “offspring”) to infect other targets. Watch the video showing the building of the 3D model based on electron microscopy images.
The images were obtained through the latest generation microscopes available at the National Institute of Metrology, Quality and Technology (Inmetro) and at the National Center of Structural Biology and Bio-Imaging of the Federal University of Rio de Janeiro (Cenabio/UFRJ). The only one of its kind in Latin America, the Inmetro equipment uses a triple ion bundle scanning technique, producing three-dimensional images with one-nanometer resolution - one nanometer corresponds to one-millionth of a millimeter. The Cenabio microscope used in the research is based on the electron transmission technique, which allows researchers to see the structures in ultrafine cuts, with a resolution of 0.45 nanometer. For comparison purposes, a paper sheet is about 100,000 nanometers thick. On average, the Sars-CoV-2 has 76 nanometers of diameter, according to measurements taken by the scientists.
The leader of the study and researcher of the Laboratory of Viral Morphology and Morphogenesis at IOC/Fiocruz, Débora Ferreira Barreto Vieira, highlights that this work consolidates important knowledge on the infection process and can contribute to the development of new treatments. “We need well-characterized in vitro models in order to develop drugs that work during different phases of the viral cycle. In this study, we described the replication cycle of the Sars-CoV-2 in Vero cells, with details and high quality”, she said.
Led by the Laboratory of Viral Morphology and Morphogenesis of the IOC/Fiocruz, the work was carried out in a partnership with the Institute’s laboratories of Measles and Respiratory Viruses, and Immunopharmacology. The research also included the collaboration of researchers from the Inmetro Hub of Microscopy Laboratories; the Center for Technological Development in Health (CDTS/Fiocruz), and the Federal University of Rio de Janeiro (UFRJ).
The infection cycle
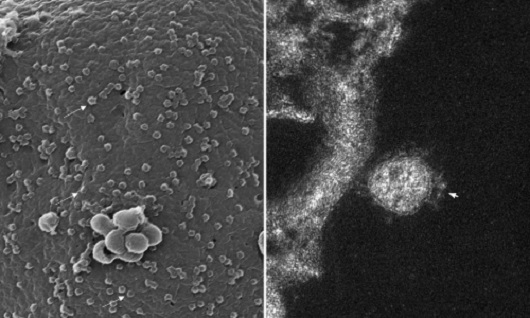
(Image: Barreto-Vieira et al; Viruses)
Nanometric enemy: After measuring more than 600 viral particles, scientists confirmed that the coronavirus has an average diameter of 76 nanometers. On the left, a three-dimensional image produced by scanning electronic microscopy, showing tens of viral particles adhered to the cellular surface (some are marked with arrows). To the right, we have a closer look at a virus adhered to the outer membrane of the cell. The image produced through transmission electron microscopy shows a slice plane of the image. We can see the spikes that led to the naming of the coronavirus, as they look like a crown.
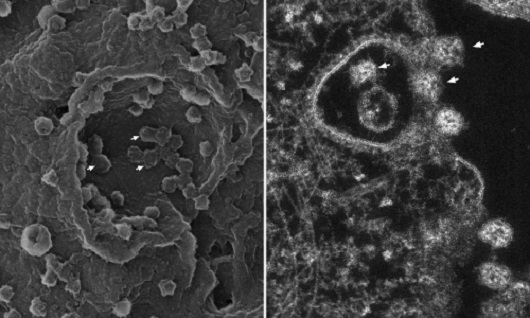
(Image: Barreto-Vieira et al; Viruses)
Cell invasion: The images show the moment the viral particles adhered to the cellular surface are embraced by the cell membrane and internalized, that is, brought to the inside of the cell, where they can then replicate. This process is called endocytosis. On the left, in the three-dimensional image obtained with the scanning electron microscope, the arrows point to some viral particles that are beginning to be enveloped by the cellular membrane. On the right, in the image obtained with the transmission electron microscope, it is possible to see the vesicle formed by the folding of the cellular membrane, with viral particles being internalized (indicated by the arrows).
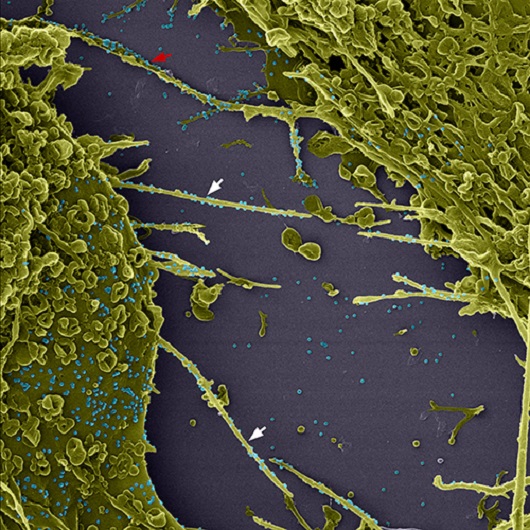
(Image: Barreto-Vieira et al; Viruses)
Delivery system and “viral surfing”: The coronavirus induces infected cells to emit membrane extensions, called filopodia, which may be used to amplify the infection in three different ways. “As the filopodium of a cell touches another cell, the viral particle that is adhered to the filopodium is carried to the neighboring cell, creating a sort of virus delivery system,” explained Vieira. As it connects with the filopodium, the coronavirus induces a rapid movement towards the body of the cell; this is known as “cell surfing.” “It’s like a track is activated, to accelerate its arrival to the body of the cell.
Other viruses, such as Sars, Ebola and Marburg, also use this mechanism,” the researcher commented. Finally, there is a possibility that the filopodium retracts, bringing the coronavirus into the cell as it does so. In the image produced through colored scanning electronic microscopy, the viral particles are displayed in blue to make visualization easier. The cells appear in green and some filopodia are marked by arrowheads. The red arrow indicates a filopodium that reaches the neighboring cell.
(Image: Barreto-Vieira et al; Viruses)
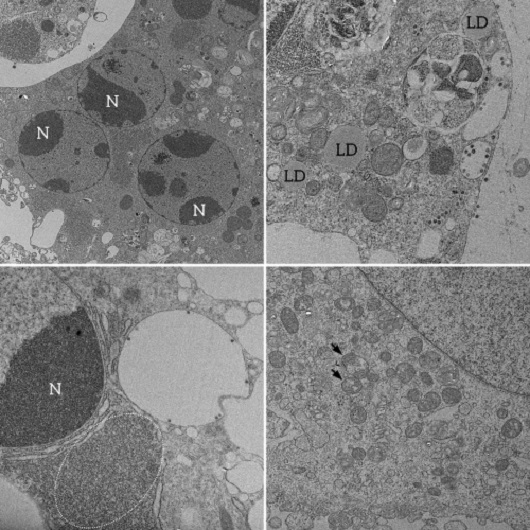
Cellular changes: Image 1 shows a cell with different nuclei (N), also called syncytium, made up of cell fusion, highlighting the loss of basic cellular architecture. Lipid drops (LD, in the Portuguese acronym), shown in image 2, are fat accumulations that may be used as energy resources necessary for the viral replication process. Mitochondria, organelles that produce energy for cellular functioning, appear thickened and containing vacuole, as shown by the arrows in image 3. This is a sign of degeneration of these organelles. The dark appearance in the area highlighted in image 4 indicates intense ribosomal activity. Ribosomes are organelles responsible for the synthesis of genetic material and proteins. During an infection, they are directed to produce viral copies. “We can see that the cell is completely active and is guided to produce viral progeny. Over time, wearing leads to cellular death,” explains Vieira. The four images were produced by scanning electronic microscopy.
(Image: Barreto-Vieira et al; Viruses)
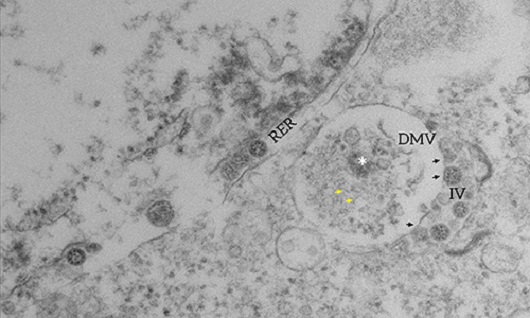
Replication compartments: Two important compartments for viral replication were caught in the images produced by electron microscopy. At the top, we can see the double-membrane vesicle (DMV); inside it there is electron-dense material (asterisk) and filaments that resemble RNA (yellow arrows), indicating ongoing genome replication activity. Derivated from a cellular organelle called rough endoplasmic reticulum (RER), the double-membrane vesicle is the main site of coronavirus replication. Next to it, we can see the intermediary vesicle (IV), where some viral particles are already formed and are indicated by black arrows.
Watch the video to see the three-dimensional model showing the interaction between the double-membrane vesicle (DMV, in red) and the intermediary vesicle (IV, in purple). Through a process called budding, the replicated viral particles (blue) cross from the double-membrane vesicle into the intermediary vesicle, which will lead the viral progeny out of the cell.
(Image: Barreto-Vieira et al; Viruses)
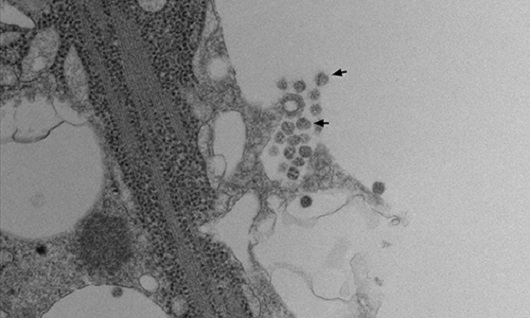
Virus release: The transmission electron microscopy image shows the exact moment when the replicated viral particles (some indicated by arrows) leave the cell. The process is called exocytosis. “The intermediary vesicles, which contain the viral progeny, migrate to the periphery of the cell, and exocytosis occurs. The infectious particles are then released to the extracellular medium, where they will propagate the infection,” says Vieira.



