Fiocruz will produce 60 million antigen tests for Brazilian public health system
17/08/2021
Pamela Lang and Ricardo Valverde (Fiocruz News Agency)
Fiocruz will take on another challenge in the fight against COVID-19 with the production and supply of approximately 60 million antigen tests to the Ministry of Health (MS, the Portuguese acronym) by the end of 2021. Tests will be provided according to the demand of the ministry.
In addition to the production, Fiocruz will also be responsible for training the teams and providing technical and scientific assistance in the locations to be defined by the MS. The initiative, which will involve an investment of approximately R$1.2 billion (US$ 227 million), will allow a faster diagnosis of the infection and is part of the testing strategy expansion in the country.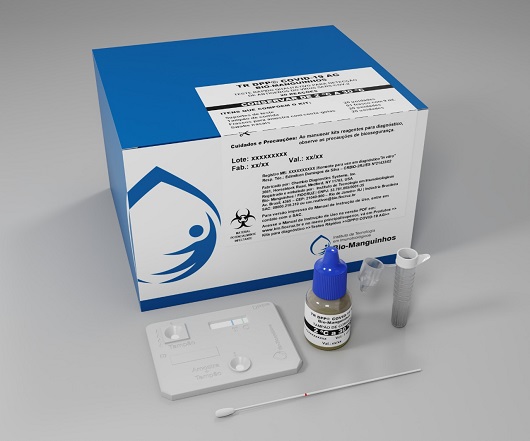
“Fiocruz has been promoting a series of activities related to the fight against the public health emergency due to COVID-19, with permanent interaction and attention to the demands made to the institution by the Ministry of Health, considering the epidemiological scenario and the new knowledge that has been produced about the disease. In this sense, Fiocruz puts its technological and production capacity at the Brazilian public health disposal again with the new diagnostic tests production”, highlighted Fiocruz’s president, Nísia Trindade Lima.
For the vice president of Production and Innovation in Health at Fiocruz, Marco Krieger, a broad testing strategy plays a key role in monitoring epidemics and their trends, being a tool to contain, slow down, and reduce the spread of the virus. “The antigen tests, combined with the molecular testing strategy, will be able to expand testing in the country, allowing the swift identification of those infected and the implementation of a strategy to track contacts of the individual who may also be infected. This way, health authorities can make a more effective and targeted intervention, detecting and isolating the infected individuals and interrupting the virus transmission chain”, explained Krieger.
The Foundation will continue to produce molecular tests (RT-PCR), considered the gold standard for virus detection. After the delivery of 11.7 million molecular tests, a new agreement was recently signed with the ministry to produce 13.7 million additional RT-PCR tests. According to Fiocruz’s specialists involved in the project, it is important to expand the number of products related to testing, such as the incorporation of the antigen test, but always having in mind an integrated strategy that considers the advantages and objectives of each one. The RT-PCR, for example, in addition to being considered the most accurate test for the disease diagnosis, is also the only one capable of sustaining a genomic surveillance action, since the variants monitoring depends on the sequencing of samples in this test type.
Antigen and RT-PCR: understand the differences
Both antigen and molecular tests serve to detect the Sars-CoV-2 virus presence during infection, but the testing methodologies follow different paths and have different sensitivity and processing times. The RT-PCR seeks to find genetic material of the virus, called RNA, and requires sending it to specialized laboratories that can process the sample and identify the virus. The processing can take several hours, relies on sophisticated equipment, and is considered the gold standard in virus detection.
Antigen testing, on the other hand, looks for specific proteins on the surface of the virus that can identify it. The collection is done in the same way as the RT-PCR, with a nasopharyngeal sample, but its processing is much faster, and a result can be obtained within 15 minutes. No sophisticated infrastructure is required and the processing can be done at the collection site itself, by mixing a solution, responsible for releasing the viral proteins, and a special paper strip that contains antibodies that will bind to these proteins in case of a positive result. The sensitivity is somewhat lower than the RT-PCR, but is still considered high, with a potential above 95%.


