Fiocruz develops a new quicker and cheaper diagnostic kit for COVID-19
12/07/2021
Maíra Menezes (IOC/Fiocruz)
A diagnostic kit to detect the new coronavirus that can be employed directly by basic health units, providing a result in up to 45 minutes, at a low cost and with high accuracy. The innovation, which may help fight COVID-19, has had its patent deposited after a year of work by researchers of the Oswaldo Cruz Institute (IOC/Fiocruz), the Federal University of Santa Catarina (UFSC), and the Federal Institute of Santa Catarina (IFSC), in a partnership with the company SPK Solutions.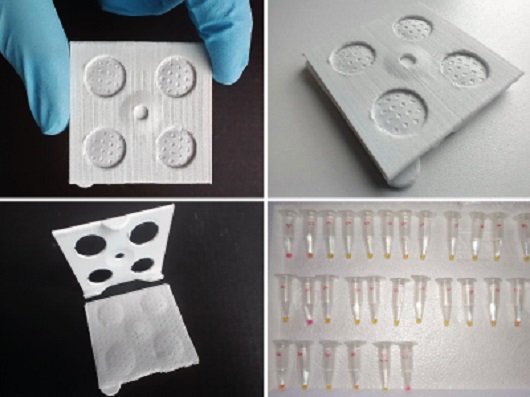
The diagnostic kit (Photo: André Pitaluga)
Simple, quick and cheap, this diagnostic kit identifies the genetic material of the Sars-CoV-2 using a technique called RT-LAMP. In validation tests with more than one thousand samples, the test has proven to be as accurate as RT-PCR, considered the gold standard for the diagnostic of COVID-19. For oropharyngeal samples, collected with a swab introduced in the patient’s nose, the test showed 96% sensitivity and 98% specificity.
“What makes this kit stand out is the fact that it integrates all the steps of the molecular diagnosis, with a methodology suitable for the point of care. This is a simple, cheap and robust method that makes it possible to make a diagnosis exactly where it is needed”, emphasizes André Pitaluga, IOC researcher and project coordinator.
The methodology can also be used for samples of saliva, which is easier to collect. In that case, the first analysis shows a 70% sensitivity and a 98% specificity, and a new round of tests shows that it is possible to achieve almost 100% sensitivity if the sample is collected while fasting, right after waking up.
“Eating, drinking or brushing one’s teeth can alter the composition of saliva and may therefore reduce the presence of viral particles in the sample, making a diagnosis harder. Tests made with the sample collected while fasting, in the morning, are still ongoing, but preliminary results are very encouraging so far”, comments the researcher.
To develop this diagnostic kit, researchers relied on the support of the IOC’s Nucleus of Technological Innovation (NIT-IOC) and on the financial support of the company Engie, of the Inova Fiocruz Program, and of the Public Ministry of Labor (MPT). Validation tests were carried out in a partnership with the Central Laboratory of Public Health of the state of Santa Catarina (Lacen-SC) and the municipalities of Tubarão and Florianópolis.
Pink or yellow
The estimated cost of the new Sars-CoV-2 diagnostic kit is US$ 5,71 (R$ 30 in Brazil), less than one-third of the price of an RT-PCR kit, which costs approximately US$ 19 (R$ 100). The operation costs to carry out the test are also significantly lower, considering the equipment and labor involved.
The researchers highlight that the tool uses components for the extraction and amplification of viral RNA. The first step aims to extract from the sample only the genetic material, leaving other molecules aside. The goal of the second step is to identify and multiply targets of the Sars-CoV-2 genome, to make its detection possible.
“Both steps are very important for the diagnosis. Extraction makes RNA detection easier, increasing the sensitivity of the test. If this procedure is not correct, the RNA of the virus suffers degradation, which can lead to a false-negative result”, highlights Luísa Rona, professor of the Cellular Biology, Embryology and Genetics (BEG) Department of UFSC and a member of the project.
The diagnostic kit includes a device for RNA extraction, created by the scientists. A simple pipette is used to deposit the solution containing the sample in the indicated place, where a membrane retains the RNA particles present in the sample.
To amplify the targets in the viral genome, the kit contains a ready-to-use solution with all the ingredients necessary for the RT-LAMP reaction, including the enzyme that participates in the process and molecules that specifically identify Sars-CoV-2 RNA. All one needs to do is to place the purified sample in a tube with the solution and use a dry bath or bain-marie device to heat up the mixture. The RT-LAMP reaction is concluded after 30 minutes at 65° C.
The result is indicated by a change in the color of the solution. Positive samples cause a change in the pH of the mixture, which then turns yellow. Negative samples remain pink.
“The entire procedure takes about 45 minutes. Due to its simplicity, its operational cost is much lower than that of RT-PCR, which requires sophisticated equipment and needs to be run by molecular biology specialists”, sums up Pitaluga.
Next steps
Based on the good results obtained in the validation tests, the researchers are now looking for partners to manufacture and supply the diagnostic kit. The project is part of the IOC’s Vitrine Tecnológica (Technological Display Window), a platform that gives visibility to innovations being developed in the Institute with the goal of promoting partnerships to generate innovative products and processes to fight this public health emergency. The next steps of the project will likely involve scaling up for industrial production, and submission to the National Agency of Sanitary Surveillance (Anvisa).




