Research points to preventive therapy against zika
22/11/2017
Matheus Carvalho da Cruz (CCS)
Widely used in the treatment of diseases like some cancers such as breast, gastric and bone, monoclonal antibodies also have high potential for application against the zika virus. The evidence was demonstrated in a study led by US scientists, in partnership with the Oswaldo Cruz Institute (IOC/Fiocruz) and the University of São Paulo (USP), published on October 4, in the international journal Science Translational Medicine. Despite the refined technique of producing monoclonal antibodies, the idea is simple: when introduced into the body, they were able to fight against zika virus, with high specificity in relation to that target.
Tests on monkeys were based on the use of monoclonal antibodies extracted from human blood in a patient in the acute stage of zika virus infection. That is: the person was producing antibodies to fight the virus and scientists needed to identify, amid the vast arsenal of immunity produced by the patient's body, which would be effective against zika. A cocktail was obtained with three monoclonal antibodies. Tests on monkeys showed that the cocktail was able to successfully block replication of zika virus - rates reached 100%.
"The method is highly promising for the prevention of congenital malformations and adverse effects on eyes and limbs, since the cocktail of monoclonal antibodies could be administered to pregnant women to prevent infection of the fetus. The scientific literature is pointing out that these proteins are extremely safe," said study leader David Watkins, an immunologist at the University of Miami." The work presents an important step in the development of a preventive action therapy against zika," said Myrna Bonaldo, head of IOC/Fiocruz Molecular Biology Laboratory of Flavivirus, who also authored the study. Diogo Magnani, who also works at the University of Miami, is the first author of the article.
Genetic engineering as an ally
To identify antibodies with the highest potential against zika it was necessary to go a long way. The first step was to perform blood collection from an individual in the acute phase of the disease. With the aid of genetic engineering, antibody producing cells were identified and antibodies were extracted from them, and 91 antibodies were isolated and purified. After in vitro tests, where monoclonal antibodies were challenged to neutralize zika virus, the three that had the highest rates of virus neutralization were selected: monoclonal antibodies called SMZAb1, SMZAb2 and SMZAb5.
The three monoclonal antibodies were administered to a group of four Rhesus monkeys. The next day, the four primates were inoculated with zika virus isolated in 2016 by Myrna and her team from a patient in Rio de Janeiro. "For this study, we used the virus that was circulating in the continent and causing an international health emergency. This fact gives more accuracy to the results," David explained. For comparison purposes, four other animals in the control group were inoculated with the virus after administration of a placebo with other antibodies that had no action against zika.
Encouraging results
To monitor the impact of the use of antibodies during the tests it was necessary to determine the levels of viral load in the animals' bodies. For this, primate blood samples were collected at 2, 3, and 7, 14 and 21 days post-infection and underwent to real-time RT-PCR technique, capable of detecting and quantifying the genetic material of the virus present in the samples. The results showed that zika-specific monoclonal antibodies were able to prevent up to 100% virus replication in the four monkeys that received the cocktail. "Meanwhile, in the group that received the placebo cocktail, we observed that all animals had a high rate of infection with zika. That is: the infection followed the course that was expected," said David Watkins.
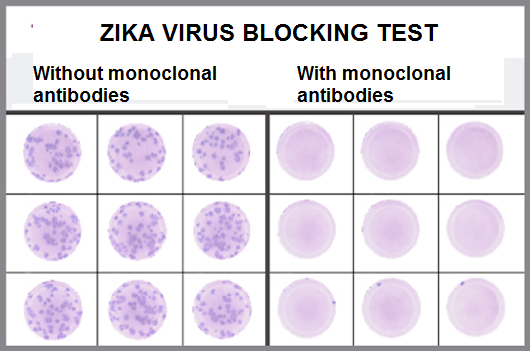
The experts went one step further. Instead of evaluating the success of the experimental therapy looking only for the presence of the virus, the scientists also made tests to detect the occurrence of specific body defense molecules that are produced in contact with some type of invading microorganism. Serological tests, applied 14 and 21 days after infection, did not show any specific response against NS1 protein produced in cases of zika virus infection in the group that received the zika antibodies. "If the production rate of NS1 antigen was zero, it is a sign that zika could not replicate in the body and invade the cells of the animals," said Myrna Bonaldo. Likewise, the production rates of IgM and IgG molecules, which indicate if the person had contact with the pathogen at some point in life, bordered on nullity. "This other result shows that zika was completely neutralized by the cocktail of monoclonal antibodies. In the scientific field, we call this sterilizing protection – when the immune response totally blocks the infection by the pathogen," the immunologist added.
Additional tests showed that monoclonal antibodies remained active and in high concentrations for almost six months in the organism of primates. "If extrapolated to human reality, this data shows that the cocktail could be recommended in cases of outbreaks of the disease to pregnant women, health professionals and other individuals who need to be or go to endemic areas. With a single dose, these groups would be protected against zika. For pregnant women, administration of the cocktail would have an extra gain as they could follow the pregnancy quietly without the daily worry that the child could be infected by zika and be affected by neurological disorders," Watkins said. "Studies have already described that the virus negatively affects 40% of pregnant women," he warned.
The American scientist estimates that the cocktail will be ready to be marketed on a large scale between two and three years, even before a zika vaccine is available for use. The next steps will be the administration of the cocktail to pregnant primates, and in the next stage, to humans.
Already predicting a possible occurrence of adverse reaction of the immune system in case of dengue infection, which is of the family Flaviviridae, as well as zika, specialists have made small modifications in the genetic structure of monoclonal antibodies to make its administration even safer. This way, the cocktail could also be used by people with a history of dengue infection, including pregnant women. "This is a shared concern for groups working on the development of dengue and zika vaccines," said David Watkins.
The discovery, which is applying for a patent filed in the United States, has also been supported by experts at the Scripps Research Institute, Emory University, the United States National Institutes of Health, and Ragon Institute.


